![]()
PSU COVID-19
Diagnostic test for the detection of SARS-CoV2 infection
- 1. Explanation of the test
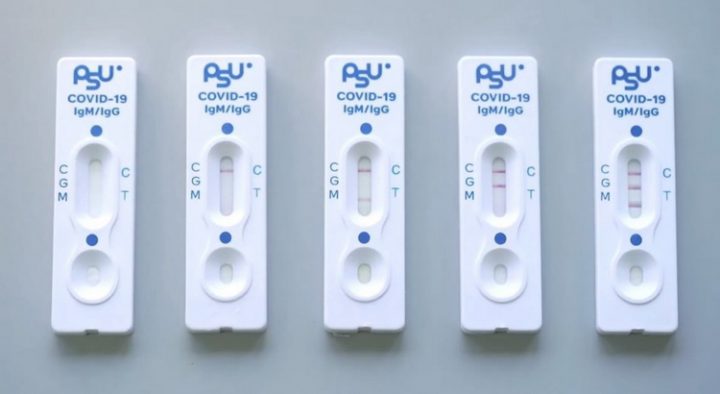
The PSU COVID-19 test is a rapid, qualitative test for the detection of antibodies to SARS-CoV-2 virus that is specific to spike protein of SARS-CoV-2 in human serum, plasma or whole blood. The PSU COVID-19 test contains colloidal gold conjugated with S1 protein of SARS-CoV-2 and colloidal gold conjugated with chicken IgY antibody, which are coated on conjugate pad. On the reaction pad (test zone), goat anti-human IgM and IgG antibodies are immobilized on IgM and IgG test lines, respectively. Goat anti-chicken IgY antibody is coated on the control line. The sample dropped onto the sample well moves along the membrane chromatographically to the test region and forms a visible line as the antigen-antibody- antibody complex forms with high degree of sensitivity and specificity. The test lines and control line in the result window are clearly labelled as “M” for the IgM test line, “G” for the IgG test line, and “C” for the control line.
In the result window shown in Figure 1, both test lines and the control line are invisible when the sample is loaded. The control line (C) is used for procedural control; it appears only when the test procedure is performed correctly.
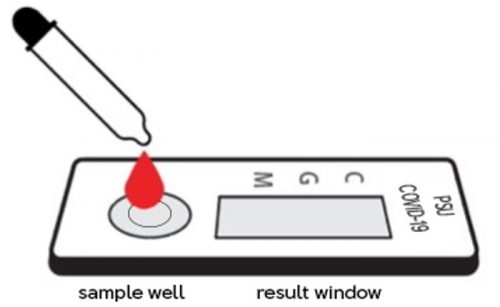
Figure 1. Test strip with the sample well and result window.
2. Provided materials
The PSU COVID-19 test kit contains the following items to perform the assay.
- Individually foil pouched test cassettes, with a desiccant (20 packs)
- Assay buffer (1 mM Tris buffer pH 8.0, Casein (0.1% w/w) and Tween80 (0.1% v/v) q.s. 2 ml (1 bottle)
- Fingerstick needles (20 pieces)
- Sample droppers (20 pieces)
- Alcohol pads (20 pieces)
- Package insert
3. Precautions
The PSU COVID-19 test devices should be stored at 4-30 °C. The test device is sensitive to humidity and heat. Perform the test immediately after removing the test device from the foil pouch. Do not use the device beyond the expiration date printed on the package.
4. Specimen Collection and Storage
[Whole Blood]
- Whole blood collected by venipuncture or by fingerstick should be collected aseptically in a way that avoids hemolysis.
- Whole blood collected by fingerstick should be tested immediately.
- If whole blood collected by venipuncture using anti-coagulant is not immediately tested, the specimen should be stored at 2 – 8 °C up to three days.
- Collect the whole blood using a suitable anti-coagulant (Heparin, EDTA, or Sodium citrate).
- Do not freeze whole blood specimens.
[Serum or Plasma]
- Human serum or plasma collected by venipuncture should be collected aseptically and the serum or plasma should be separated from blood as soon as possible, in a way that avoids hemolysis. Only clear, non-hemolyzed specimens can be used.
- If specimens are not immediately tested, they should be refrigerated at 2 – 8°C. For storage period longer than three days, freezing is recommended. The specimens should be brought to room temperature prior to use.
- Specimens containing precipitate may yield inconsistent test results. Such specimens must be clarified prior to assaying.
5. Warnings
- For in vitro diagnostic use only.
- The kits should be used by medical staff or trained personnel only.
- Do not eat, drink or smoke while handling the specimen.
- Wear protective gloves while handling the specimen.
- Avoid splashing or aerosol formation.
- Clean up spills thoroughly using an appropriate disinfectant.
- Decontaminate and dispose of all specimens, reaction kits and potentially contaminated materials in a biohazard container.
- Do not use the test device if the pouch is damaged or if the seal is broken.
- Do not use any of the components beyond expiration date.
6. Procedure of the test
- Remove the test device from the foil pouch and place it on a flat and dry surface.
- Add 5-10 μl or 1 drop of serum, plasma (10-15 μl or 1-2 drops of whole blood) by sample dropper to the sample well (S) of the test device, and then add 3 drops of assay buffer (approximately 100 μl) and start timer.
- As the test works, a flow of red-purple color across the result window in the center of the test device is noticeable.
- Read the results in 15-20 minutes.
*Caution: If the test result is not legible after 20 minutes due to high background color, read again later but within 30 minutes after adding the sample buffer. Do not read after 30 minutes.
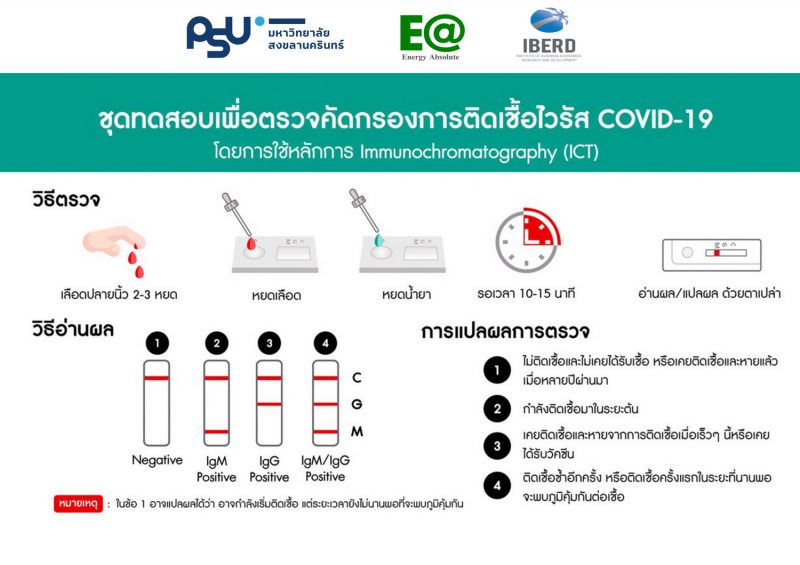
7. Interpretation of the test
The test can result in five possible combinations of patterns
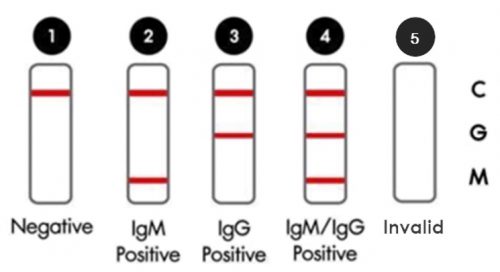
Figure 2. Five patterns of the result
- Negative
The presence of only one control line (“C” band) within the result window indicates a negative result.
Interpretation: Not infected, or very early infection with undetectable antibodies.
- IgM positive
The presence of two colored lines (“C” and “M” bands) within the result window indicates an IgM positive result.
Interpretation: Currently infected, with detectable antibodies.
- IgG positive
The presence of two colored lines (“C” and “G” bands) within the result window indicates an IgG positive result.
Interpretation: Had been infected and recovered from an infection recently, or has been vaccinated.
- IgM/ IgG positive
The presence of three colored lines (“C”, “M”, and “G” bands) within the result window indicates an IgM/ IgG positive result.
Interpret: Secondary infection or prolonged infection with two types of covid-19-specific antibodies.
- Invalid
If the red-purple color of control band (“C”) is not visible within the result window after performing the test, the result is considered invalid.
Interpret: Invalid result. Repeat the test with a new device. It is recommended to check a package integrity and the expiry date.
*Caution: The PSU COVID-19 test kit is a screening test. The sample that gives a positive result must be confirmed by SARS-CoV-2 RT-PCR (reverse transcriptase polymerase chain reaction). A negative result does not exclude the possibility of SARS-CoV-2 infection. The patient should be followed up and should repeat the test again after 7 days.
8. Limitations of the test
- A negative result does not rule out SARS-CoV-2 infection. The specimen may contain low level of antibodies to SARS-CoV-2, or the patient is in an early stage of infection with undetectable antibodies.
- The positive specimens should be confirmed by SARS-CoV-2 RT-PCR.
- Anti-SARS-CoV-2 testing alone cannot be used to diagnose COVID-19.
9. Performance characteristics
1) Sensitivity and Specificity
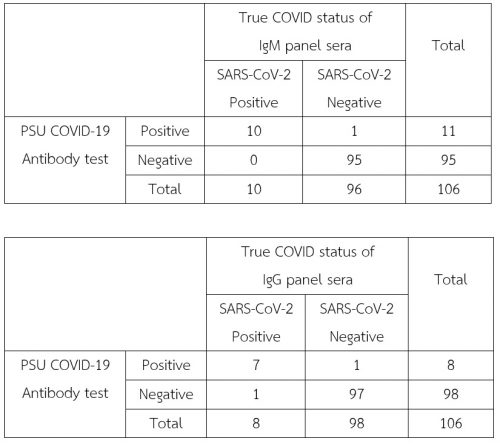
A total of 106 true COVID-19 status specimens were tested by PSU COVID-19 kit at multi-site evaluation centers. The PSU COVID-19 demonstrates a sensitivity of 100% (10/10) and a specificity of 99.8% (95/96) for anti-SARS-CoV-2 IgM antibody detection and demonstrates a sensitivity of 87.5% (7/8) and a specificity of 99.8% (97/98) for anti-SARS-CoV-2 IgG antibody detection.
2) Precision
(1) INTRA RUN: the reproducibility was determined by testing three different replicates of four different specimens containing different concentrations of antibody with three different lots PSU COVID-19 simultaneously. The precision was determined to be 100%.
(2) INTER RUN: the reproducibility was determined in different days by testing 10 replicates of four different specimens containing different concentrations of antibody with SARS-CoV-2. The precision was determined to be 100%.
10. Bibliography and suggested reading
– Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., Zhang Y., Wang J., Huang B., Lin Y., Yang J., Cai W., Wang X., Cheng J., Chen Z., Sun K., et al. (2020). Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. Journal of Medical Virology. Advance online publication. https://doi.org/10.1002/jmv.25727
Manufactured by
KESTREL BIO SCIENCES (THAILAND) COMPANY LIMITED
ISO13485
ข่าวอ้างอิง :
http://www.securitysystems.in.th/2020/05/psu-covid-19-rapid-test/
http://www.securitysystems.in.th/2020/06/psu-covid-19-rapid-test-launching-press-conference/